Abstract
Introduction:
Primary gastrointestinal (GI) T-cell lymphoproliferative diseases (T-LPD) are heterogeneous entities, which diagnoses and therapeutic options remain to be standardized. Beside aggressive lymphoma, such as enteropathy-associated T-cell lymphoma (EATL) and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL), the indolent T-cell lymphoproliferative disease (indolent T-LPD) has been recently described. A differential diagnosis to consider is type II refractory coeliac disease (RCDII) that may complicate CD. Intraepithelial lymphocytes (IEL) from RCDII are dependent for survival on IL-15, which reprograms T-lymphocytes towards a cytotoxic NK phenotype. We previously showed that NKp46 is a new biomarker for both diagnosis and prognosis in GI T-LPD (ICML-2017 Lugano, unpublished).
Patients and Methods:
The expression of NK receptors was assessed on CD/RCD by flow-cytometry. The number of NKp46 positive IEL per 100 epithelial cells (NKp46+ IEL/100 EC) was studied by immunohistochemistry (IHC) on 195 biopsies from 84 CD/RCD, 44 GI T-cell lymphoma, and on a blinded confirmatory cohort of 48 CD/RCD.
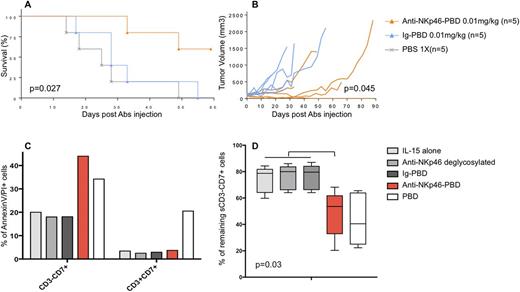
8B6A-PBD is an anti-NKp46 monoclonal antibody conjugated (ADC) to a potent cytotoxic drug, pyrrolobenzodiazepine (PBD). For in vivo assessment, tumor-bearing NOD-SCID mice were randomly assigned to PBS, isotype-matched irrelevant Ig-PBD controls, or 8B6A-PBD treatment groups. For ex vivo cytotoxicity assays, isolated cells from fresh small bowel biopsies of RCDII patients were incubated with 8B6A-PBD, Ig-PBD, anti-NKp46 deglycosylated or PBD alone.
Results:
NKp46 was the only NK receptor that differs between RCDII and CD or RCDI. By doing ROC analysis, a number of NKp46+ IEL/100 EC above 25 clearly discriminated RCDII from CD and RCDI, with excellent positive and negative predictive values of 100 and 95% respectively. Among CD and RCD patients, the survival was shorter if this count is above 25 (OS-5years 72.8% vs. 96.4%, P=0.0004). NKp46 was expressed in EATL (n=20/25) and MEITL (n=4/4) but was never expressed in indolent T-LPD (n=0/15). Positive NKp46 GI T-cell lymphoma had a shorter survival compared to negative NKp46 cases (OS-5years 5.4% vs. 50.5%, P=0.0011).
In vivo the anti-NKp46-PBD promoted tumor regression (Fig. B) and prolonged survival (Fig. A) of NOD-SCID mice injected by human NKp46-transfected Raji tumors embedded in matrigel. Ex vivo and as a proof of concept, the anti-NKp46-PBD was highly potent (Fig. D) and selective (Fig. C) against NKp46 positive primary sorted IEL (n=5).
Conclusion:
NKp46 provides a new biomarker for both diagnosis and prognosis in GI T-LPD. We propose a diagnostic algorithm for GI T-LPD based on the NKp46 expression, which is adapted to immediate, individual patient management. Overall, our results demonstrate for the first time that anti-NKp46 ADC may serve as an effective therapy for RCDII, EATL and MEITL and provide strong preclinical rationale for evaluation of 8B6A-PBD in clinical studies.
Cheminant: Innate Pharma: Research Funding. Bruneau: Innate Pharma: Research Funding. Malamut: Cellimmune: Research Funding. Pouyet: MI-mAbs: Employment. Lhospice: Innate Pharma: Employment. Molina: Merck Serono: Honoraria; Takeda: Other: travel support to the ASH meeting; Novartis: Honoraria. Cellier: Cellimmune: Research Funding. Cerf-Bensussan: Cellimmune: Research Funding. Hermine: Novartis: Research Funding; Celgene: Research Funding; AB Science: Equity Ownership, Honoraria, Patents & Royalties, Research Funding; Hybrigenics: Research Funding; INatherys: Equity Ownership, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal